The Role of Steam
In modern papermaking, one of the most effective methods for removing water from the sheet is through the utilization of steam’s latent heat of vaporization. This principle is fundamental to efficient drying operations and remains a cornerstone of process optimization in the industry. Steam is not only one of the most valuable resources in paper production, but one of the most dependable as well.
Steam offers distinct advantages over alternative heating media. It carries a high concentration of usable thermal energy, distributes heat uniformly, and delivers consistent, controllable performance. These characteristics make steam ideally suited to the required thermal management in the drying section of the paper machine. A clear understanding of its properties, and its role in the steam circuit, is essential for operating and maintaining efficient steam systems.
Understanding Steam: From Water to Vapor and Back
Steam is water that has undergone a phase change from liquid to vapor through the application of thermal energy. At the molecular level, water (H₂O) is comprised of hydrogen and oxygen atoms bonded together. In the liquid state, these molecules remain in close proximity due to intermolecular forces.
As thermal energy is applied, molecular motion intensifies. At atmospheric pressure, water reaches its boiling point at 212° F (100° C). At this point, the average kinetic energy of the molecules is sufficient to overcome intermolecular attraction. With continued thermal input, the molecules separate to form vapor, a transition visible as bubbles rising through the liquid during boiling.
Sensible Heat
Sensible heat is the energy required to raise the temperature of water to its boiling point without initiating a phase change. At standard atmospheric pressure, raising water from 60° F to 212° F (16° C to 100° C) requires approximately 152 BTU per pound (212 – 60 = 152). During this process, the liquid is brought to the boiling point but does not undergo a phase change. In summary, sensible heat is energy absorbed or released in a body where the temperature of the body raises or lowers.
Latent Heat of Vaporization
Once water reaches its boiling point, additional heat input does not increase temperature but instead drives the phase change from liquid to vapor. This energy is defined as the latent heat of vaporization. At standard atmospheric pressure, the latent heat of vaporization for water is approximately 970 BTU per pound. In summary, latent heat is energy absorbed or released in a body where the body undergoes a change in phase or state.
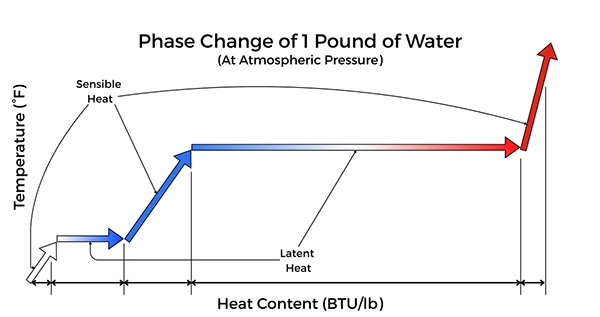 Figure 1: Phase Change of 1 Pound of Water (at ATM)
Figure 1: Phase Change of 1 Pound of Water (at ATM)
Steam Life Cycle in a Paper Mill
In industrial processes, steam is generated under pressure higher than at atmospheric conditions. This alters the boiling point and associated thermodynamic properties. In the boiler house, thermal energy is applied to water, producing steam that is carried through the main steam header to the dryer sections of the paper machine.
For example, in a header operating at 150 psig, saturated steam is distributed to steam groups in the dryer section, where it passes through the rotary joints and fills the volume of each dryer cylinder. Upon contacting the dryer shell, the steam condenses, releasing its latent heat through the shell into the sheet. The energy transfer drives evaporation of water from the paper web, advancing the drying process.
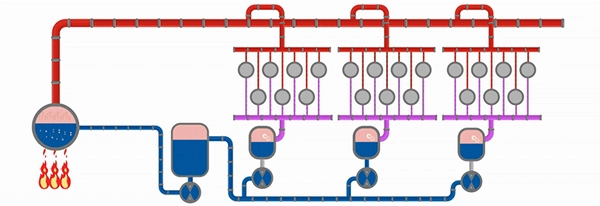 Figure 2: The Steam Circuit in Paper Manufacturing
Figure 2: The Steam Circuit in Paper Manufacturing
Dryer Sections and Condensate Removal
Efficient operation of the dryer section requires the removal of condensate. A buildup of excessive condensate acts as an inhibitor to heat transfer. A syphon system ensures adequate clearance between the syphon foot and the dryer shell, allowing evacuation of the condensate along with a portion of blow-through steam. This bi-phase flow travels through the syphon leg, rotary joint, and into the condensate header.
The bi-phase mixture is directed to a separator tank, where blow-through steam is separated from condensate. The steam may be cascaded to a lower-pressure steam group or recirculated within the same section via a thermocompressor loop. The condensate collected at the bottom of the separator tank is pumped back to the boiler house, where it's reused to make steam, thereby completing the circuit.
About the Author
 Seth Niece
Seth Niece
Capital Sales Manager
Kadant Johnson LLC

























































